
Trajectories of Recovery after Intravenous Propofol vs. Inhaled VolatilE Anesthesia
This is a multi-site, randomized controlled trial, which compares patient outcomes and experiences under the two most commonly used forms of general anesthesia: propofol TIVA (total intravenous anesthesia) and inhaled volatile anesthesia (INVA).
Both of these types of general anesthesia have been used safely worldwide for decades. However, we do not know which anesthetic type results in better patient recovery after surgery.
THRIVE Background
The Study
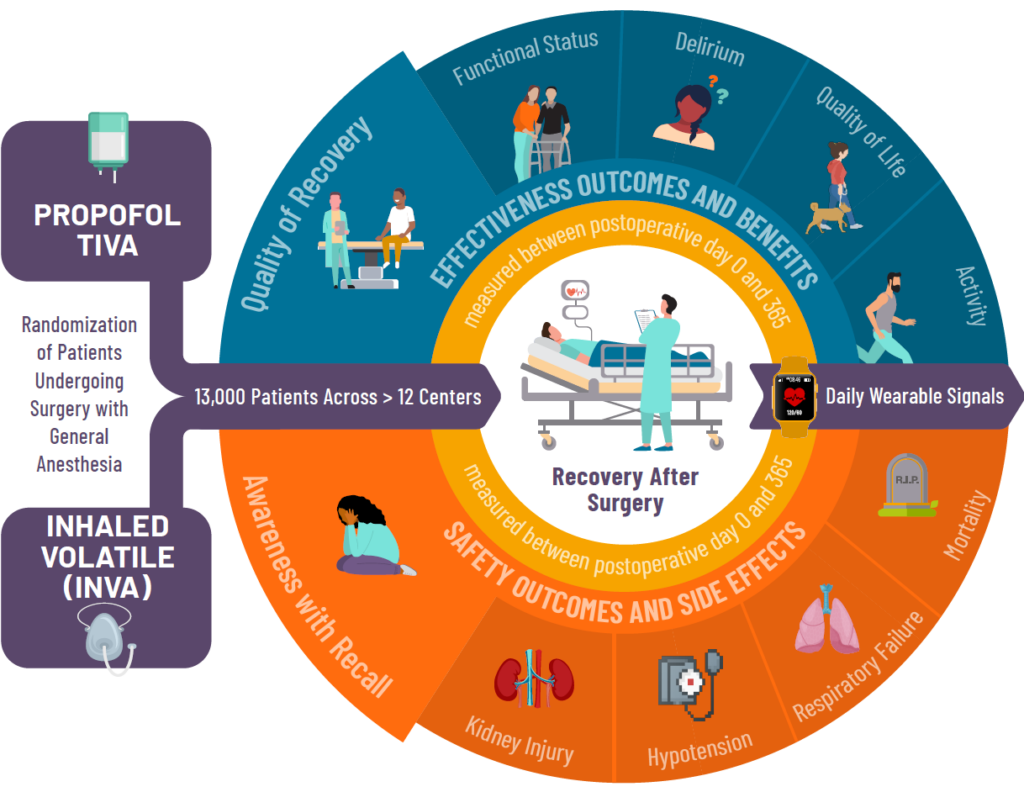
THRIVE is a multicenter, patient-level, pragmatic comparative effectiveness, randomized control trial evaluating the superiority of propofol total intravenous anesthesia (TIVA) over inhaled volatile general anesthesia (INVA). This initiative is supported by the Patient-Centered Outcomes Research Institute (PCORI).
The purpose of the THRIVE research study is to compare the two types of anesthesia in order to determine which choice results in a better patient experience of recovery and outcomes.
The study is led by Co-Principal Investigators, Michael Avidan, MBBCH, FCASA of Washington University in St. Louis and Sachin Kheterpal, MD, MBA of the University of Michigan.
- Study Directors:
- Bethany Pennington, PharmD, BCPS
- Douglas Colquhoun, MBChB, MSc, MPH
- Allison Janda, MD
- Mark Neuman, MD, MSc
- Program Mangers:
- Laura Swisher, MS
- Chelsea Cloyd, MBA
- Mara Bollini, MHA, BSN, RN, CPPS
- Shelley Vaughn, MPH
- Nicole Eyrich, MPH
Why is it being done?
By comparing propofol TIVA and inhaled volatile anesthesia, the THRIVE team hopes to improve patient outcomes by determining whether one of the two tested general anesthesia techniques benefits quality of recovery and functional status, while assessing any impact on risk of delirium, intraoperative awareness with recall, kidney injury, respiratory failure and mortality.
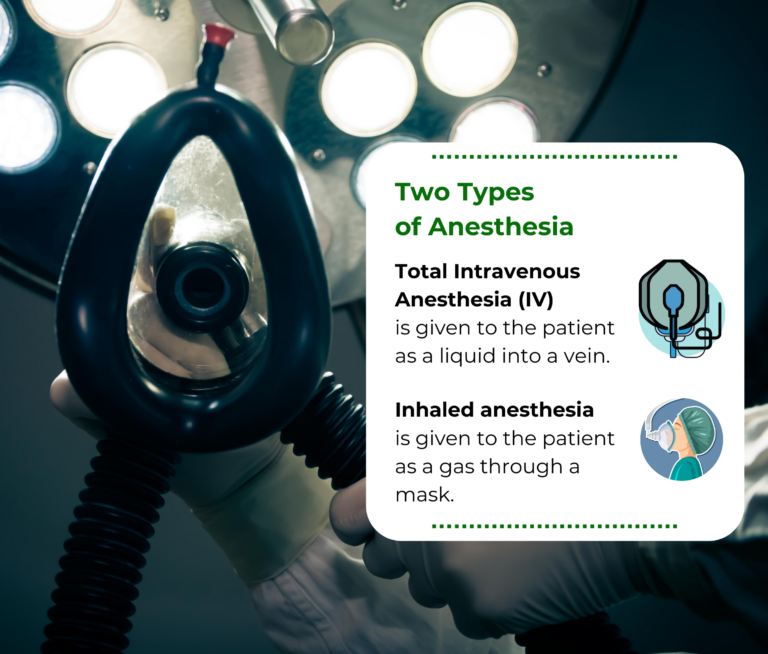
How The Study Works
Adults 18 years of age or older, having elective surgery not involving the heart and will require having general anesthesia with unconsciousness for their surgery may be eligible for this study.
Study participants will be randomized to either of the two types of general anesthesia. Participants will be given a series of short surveys both before and after their surgery. Each survey requires a time commitment of 2-3 minutes and will involve questions about:
- Overall well-being
- Mood
- Activities of daily living
- Confusion
- Memories of waking during surgery
Patient reported survey data is collected up to 1-year postoperatively.
Project Timeline
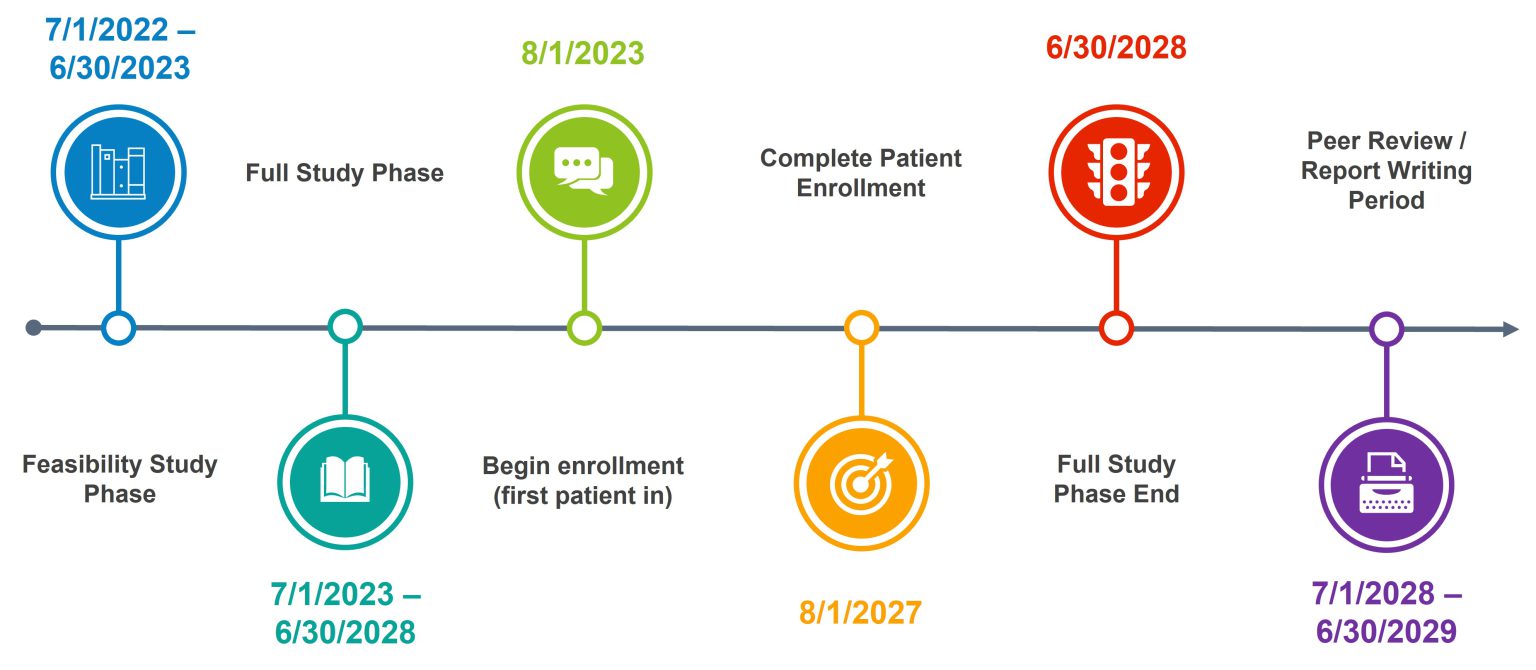
Participants enrollment is planned for 4 years with follow-up surveys being completed up to 1 year postoperatively leading to a planned trial duration of 5 years
THRIVE Engagement

Patient & Caregiver Engagement
Patient and caregiver partnership is essential to keep THRIVE thriving. The recruitment, retention, and engagement of eligible patients is essential for THRIVE’s success. A central structure of patient and caregiver partners drives the patient and caregiver voice across THRIVE as members of the research team.
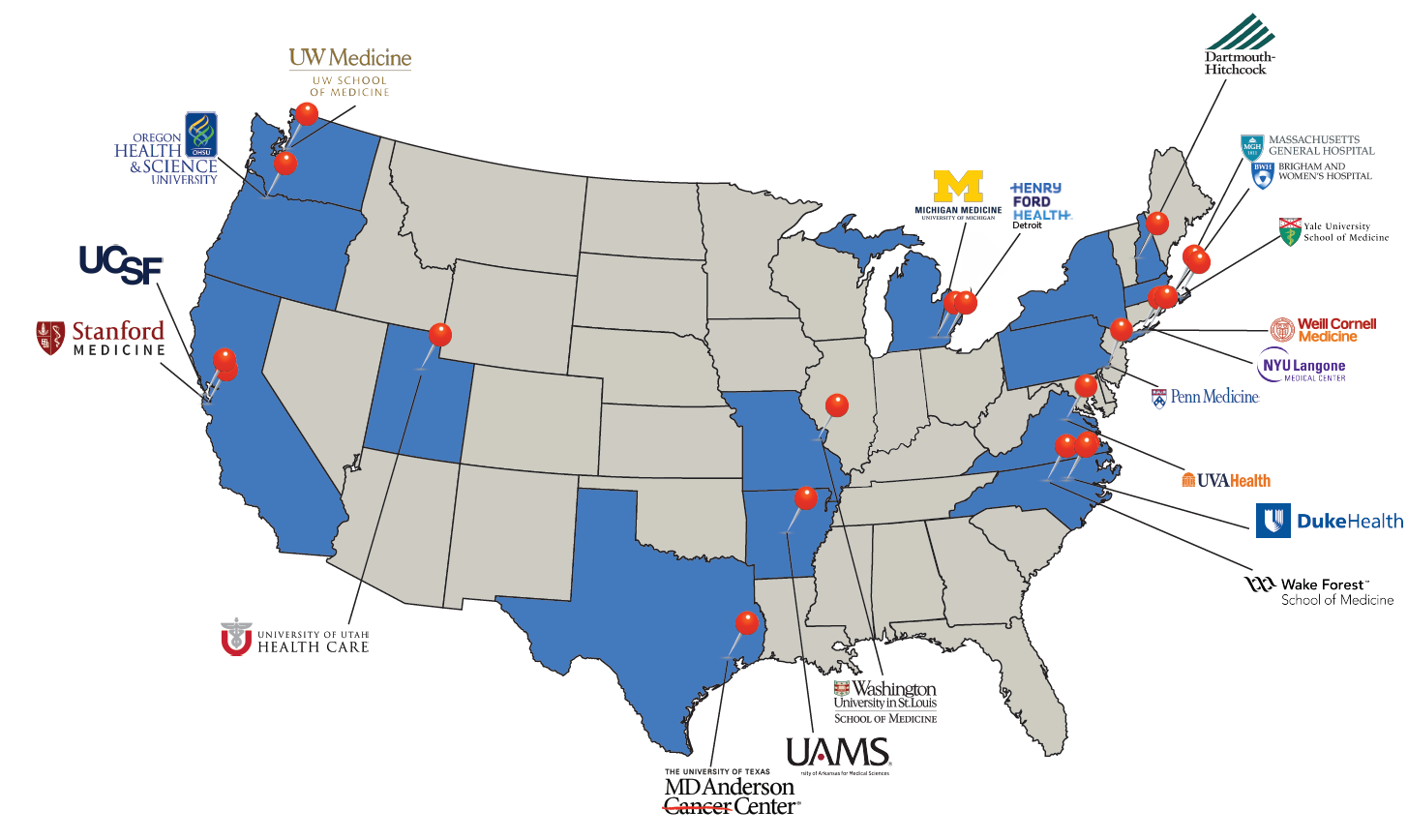
Participating Institutions
The success of THRIVE sites in recruiting a population of patient participants that represents their communities is paramount to translating study results across the country.

Stakeholder Engagement
The national healthcare landscape plays an instrumental role in influencing policy and driving practice. As a result, key stakeholders across the field of anesthesiology, surgery, and advocacy groups for equitable care across populations have been called on as partners of THRIVE.
Institution | Primary Investigator |
Brigham and Women’s Hospital | Luigino Nascimben, MD |
Dartmouth-Hitchcock | Stacie Deiner, MD, MS |
Duke University Hospital | Kamrouz Ghadimi, MD |
Henry Ford Detroit | Anoop Chhina, MD |
Massachusetts General Hospital | Amit Bardia, MBBS |
MD Anderson Cancer Center | Juan Cata, MD |
NYU Langone Medical Center | Simon Tom, MD |
Oregon Health & Science University | Mike Aziz, MD |
Stanford University | David Drover, MD |
University of Arkansas | Geoff Muller, MD |
University of California San Francisco | Lee-lynn Chen, MD |
University of Michigan | Allison Janda, MD |
University of Pennsylvania | Caoimhe Duffy, MD |
University of Utah | Emily Drennan, MD |
University of Virginia | Bhiken Naik, MD |
University of Washington | Karen Domino, MD |
Wake Forest University | Ashish Khanna, MD |
Washington University in St. Louis | Stephen Gregory, MD |
Weill Cornell Medicine | Kane Pryor, MD |
Yale School of Medicine | Jaime Hyman, MD |
Deputy Editor & Editorial Director for Equity for JAMA | Preeti Malani, MD, MS, MSJ |
ASA – American Society of Anesthesiologists | Don Arnold, MD, FACHE, FASA |
APSF – Anesthesia Patient Safety Foundation | Dan Cole, MD |
BCBSM – Blue Cross Blue Shield of Michigan | Fari Amad, Md, MBA, FACOG |
AANA – American Association of Nurse Anesthesiology | Maria Van Pelt, PhD, CRNA, FAAN, FAANA |
AUA – Association of University Anesthesiologists | Julie Freed, MD, PhD |
IARS – International Anesthesia Research Society | Beverly Orser, MD, PhD, FRCPC |
SBAS – Society of Black Academic Surgeons | Dineo Khabele, MD |