
Trajectories of Recovery after Intravenous Propofol vs. Inhaled VolatilE Anesthesia

THRIVE is a research study that aims to compare how patients recover after receiving two of the most common types of general anesthesia during surgery.
General anesthesia has a long history of safe and effective medical use. Today, total intravenous anesthesia (TIVA) and inhaled volatile anesthesia are the most common anesthetic techniques.
Both types of anesthesia are considered safe and are widely used. However, we still have questions about how each method might affect a patient’s recovery. The purpose of the THRIVE study is to compare these two types of anesthesia to determine which one leads to a better patient recovery experience.
THRIVE Background
The Study
THRIVE is a type of research study called a pragmatic comparative effectiveness randomized control trial. A pragmatic trial means that instead of testing treatments in a strictly controlled, artificial environment, we want to know how these treatments work in real-world care, with real doctors and real patients in a variety of hospitals. Comparative effectiveness means we are comparing two different treatments that are already in use, rather than testing something completely new. Participants are assigned to one of the two treatments by randomization (like flipping a coin) so that we can fairly see how each one works.
The study is led by Co-Principal Investigators Michael Avidan, MBBCH, FCASA, of Washington University in St. Louis, and Sachin Kheterpal, MD, MBA, of the University of Michigan.
Two Types of Anesthesia
There are several types of anesthesia used in medical care. In this study, we are comparing two of the most commonly used methods of general anesthesia:
Total intravenous anesthesia uses a medication called propofol, which has been safely used since the 1980s. Propofol is given through an IV (a vein) and works quickly with few side effects.
Inhaled volatile anesthesia uses modern versions of older medications such as ether. The patient breathes these medications in and out while under anesthesia.
Why Is It Being Done?
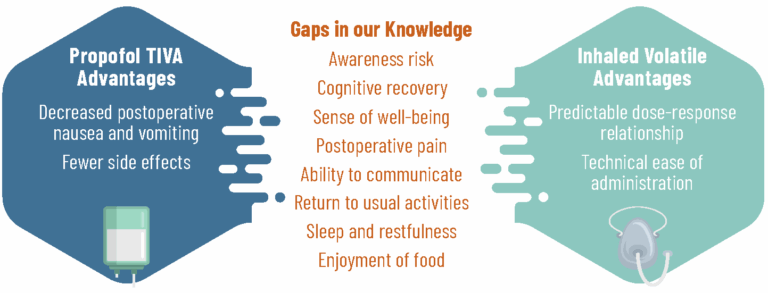
While we know some things about how patients recover after anesthesia, there are still gaps in our knowledge. With this study, we aim to learn which type of anesthesia helps patients return to their usual activities sooner, better supports their cognitive recovery — including thinking and memory — and their pain after surgery.
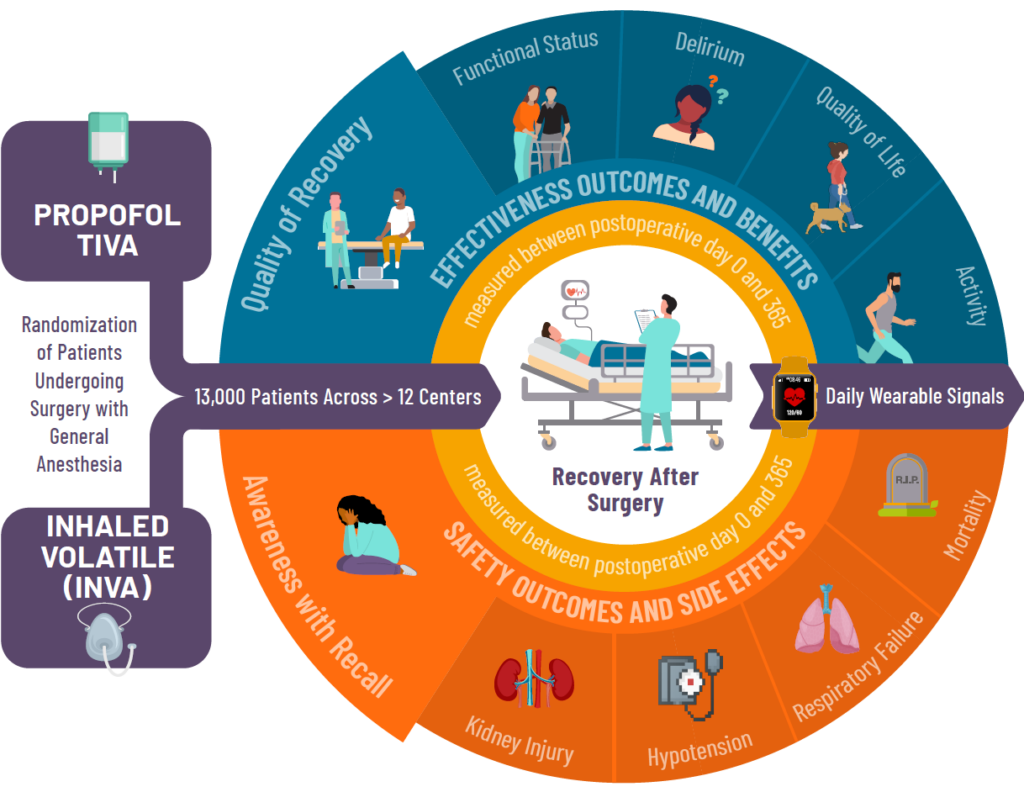
How The Study Works
Adults 18 years of age or older, having scheduled surgery not involving the heart, that will require having general anesthesia with unconsciousness for their surgery may be eligible for this study.
Study participants will be randomly assigned to receive one of the two types of general anesthesia. Participants will be asked to complete a series of short surveys before and after their surgery. Each survey takes about 2-3 minutes and will involve questions about:
- Overall well-being
- Mood
- Daily Activities
- Confusion
- Any memories of waking up during surgery
These surveys will be given up to 1-year after surgery.
Project Timeline
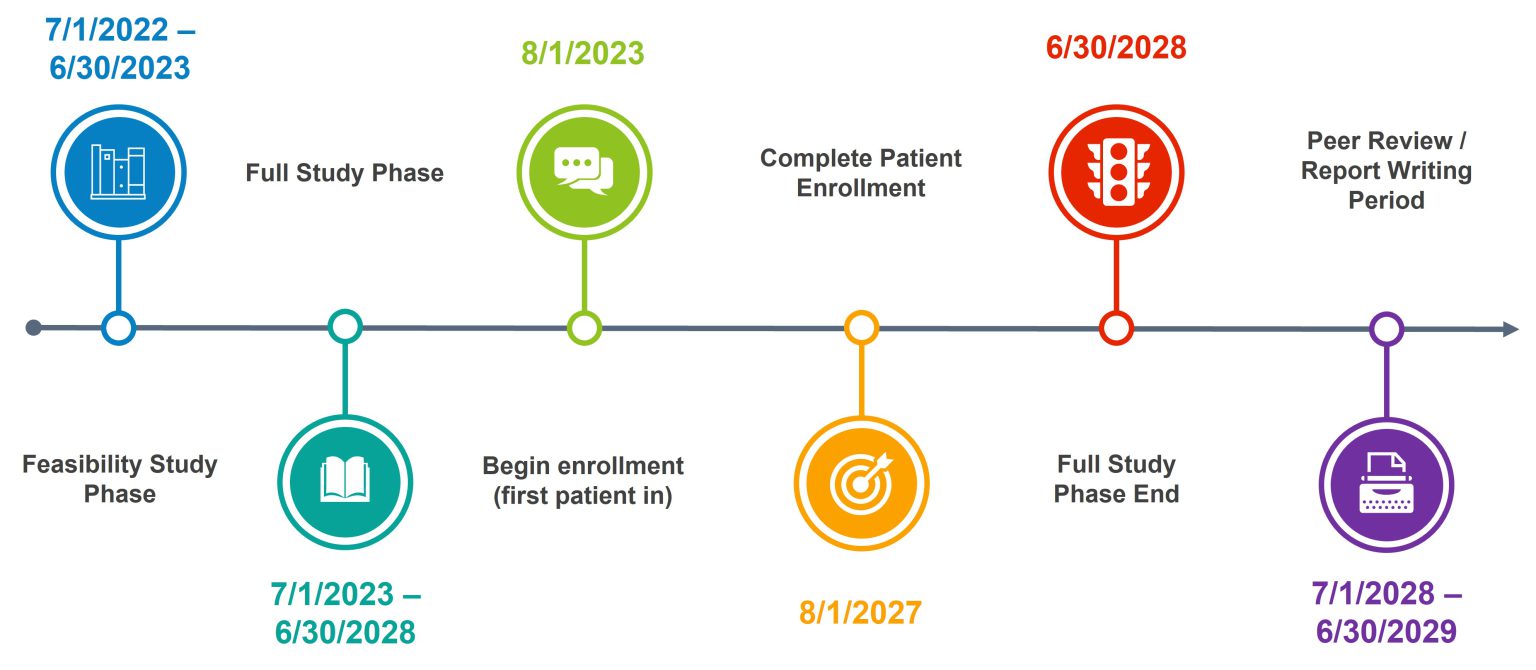
Participant enrollment is planned for 4 years with follow-up surveys completed up to 1 year after surgery, leading to a trial duration of 5 years.
The THRIVE Team
The people involved in making the THRIVE study possible work at leading hospitals and institutions across the country. Study directors and program managers at the coordinating centers — Washington University in St. Louis, University of Michigan, University of Pennsylvania and Stanford University — oversee the study and guide our research activities. Study teams at over 20 hospitals recruit patients to participate in the study.
Study Directors and Program Managers
Study Directors
- Douglas Colquhoun, MB ChB, MSc, MPH
- Allison Janda, MD
- Mark Neuman, MD MSc
- Bethany Pennington, PharmD, BCPS
Program Managers
- Mara Bollini, MHA, BSN, RN, CPPS
- Chelsea Cloyd, MBA
- Nicole Eyrich, MPH
- Laura Swisher, MS
- Shelley Vaughn, MPH
Participating Hospitals and Site Principal Investigators
- Brigham and Women’s Hospital, Luigino Nascimben, MD
- Dartmouth-Hitchcock, Stacie Deiner, MD, MS
- Duke University Hospital, Kamrouz Ghadimi, MD
- Henry Ford Detroit, Anoop Chhina, MD
- Massachusetts General Hospital, Amit Bardia, MBBS
- MD Anderson Cancer Center, Juan Cata, MD
- NYU Langone Medical Center, Simon Tom, MD
- Oregon Health & Science University, Mike Aziz, MD
- Stanford University, David Drover, MD
- University of Arkansas, Geoff Muller, MD
- University of California San Francisco, Lee-lynn Chen, MD
- University of Michigan, Allison Janda, MD
- University of Pennsylvania, Caoimhe Duffy, MD
- University of Utah, Emily Drennan, MD
- University of Virginia, Bhiken Naik, MD
- University of Washington, Karen Domino, MD,
- Wake Forest University, Ashish Khanna, MD
- Washington University in St. Louis, Stephen Gregory, MD
- Weill Cornell Medicine, Kane Pryor, MD
- Yale School of Medicine, Jaime Hyman, MD
Engagement and Collaboration

Patient & Caregiver Engagement
Patient and caregiver partnership is essential to keep THRIVE thriving. A central structure of patient and caregiver partners drives the patient and caregiver voice across THRIVE as members of the research team. Our patient and caregiver partners regularly review documents for participants in the study and advise the central study team on study processes.

Stakeholder Engagement
The national healthcare landscape plays an instrumental role in influencing policy and driving practice. As a result, key stakeholders across the field of anesthesiology, surgery, and advocacy groups for equitable care across populations have been called on as partners of THRIVE.
Michigan Medicine – Department of Medicine, Division of Infectious Diseases | Preeti Malani, MD, MS, MSJ |
American Society of Anesthesiologists – ASA | Don Arnold, MD, FACHE, FASA |
Anesthesia Patient Safety Foundation – APSF | Dan Cole, MD |
Blue Cross Blue Shield of Michigan – BCBSM | Faris Ahmad, MD, MBA, FACOG |
Bouvé College of Health Sciences, Northeastern University | Maria Van Pelt, PhD, CRNA, FAAN, FAANA |
Association of University Anesthesiologists – AUA | Julie Freed, MD, PhD |
International Anesthesia Research Society – IARS | Beverly Orser, MD, PhD, FRCPC |
Society of Black Academic Surgeons – SBAS | Dineo Khabele, MD |
Funding
The THRIVE trial is funded by the Patient-Centered Outcomes Research Institute (PCORI). PCORI is a non-profit organization that funds projects designed to answer important clinical questions that are most relevant to patients. It deeply values patient input and requires that patients act as partners throughout the process of study design and implementation.
The THRIVE Study is a clinical trial, so it is listed on ClinicalTrials.gov. This website is a database of clinical research studies and their results.
If you are a patient currently participating in THRIVE, please see your consent document for your local hospital’s study contact information.
For all other inquiries, please email askthrive@umich.edu